Manufacturing Shift Lead
Singapore, Singapore Job ID R0157136 Category Manufacturing & Supply Subcategory Manufacturing & Supply Business Unit Global Manufacturing & Supply Job Type Full timeBy clicking the “Apply” button, I understand that my employment application process with Takeda will commence and that the information I provide in my application will be processed in line with Takeda’s Privacy Noticeand Terms of Use. I further attest that all information I submit in my employment application is true to the best of my knowledge.
Job Description
About the role:
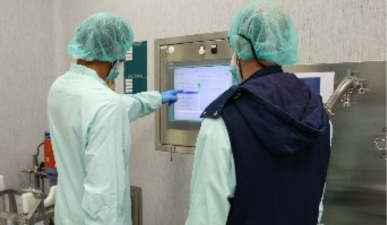
This position is required perform with minimal supervision the individual will perform routine and critical manufacturing operations, including but not limited to work functions in Cell Culture and Purification areas. Operates production equipment according to Standard Operating Procedures (SOPs) for the production of commercial and/or clinical products. Will support or lead engineering and validation activities. Deputy to the Shift Supervisor, acting on Supervisor’s behalf when needed. The individual will deliver excellence in manufacturing processing and provide support in integrate best practices, where appropriate, into manufacturing.
How you will contribute:
Primary Responsibility
Execution of all routine and critical operations in both Upstream and Downstream or both Equipment Prep and Buffer Prep areas. Perform commissioning and validation activities in respective areas.
Active participation in troubleshooting. Collaborate with Shift Supervisor, SME and Manufacturing Leadership for issue resolution.
Serve as deputy for Shift Supervisor and acting Supervisor during Supervisor absences
Monitor material consumption and coordinate all materials deliveries
Learn and perform well-defined SOPs in all areas of responsibility
Pursue on-the-job training through Competency Assessments to increase knowledge and understanding
Execute instructions and record data in the Electronic Batch Management (EBM) system if applicable
Execute instructions and record data in the Master Batch Records (MBRs) and Master Formulation Records (MFRs) if applicable
Review manufacturing documentation and EBM or PCS alerts real-time to ensure compliance if applicable
Attain operating knowledge of the Process Control System (PCS)
Record data into logbooks and log-sheets
Review logbooks and log-sheets data
Ensure documentation is complete, reviewed and meets good documentation practices (forms, logbooks, EBM, batch records, etc.)
Perform equipment monitoring
Perform and coordinate basic laboratory tasks including but not limited to sampling, pH and conductivity measurements
Demonstrate aseptic technique in the handling of product and materials
Informing management of events impacting production schedule
Propose and review document revisions
Recommend/Implement process changes/improvements or safety/ergonomic improvements.
Complete required training on time
Carry out work in a safe manner, notifying management of safety issues and risks
Quality
Lead and participate in investigations of production events in the Global Event Management System (GEMS) or equivalent systems and work with cross-functional departments to identify root causes.
Complete action items for event investigations
Implement appropriate CAPAs from event investigations
Review document revisions of Standard Operating Procedures/Batch Records
Communicate any quality issues/concerns to Supervisor and QA
Implement Change Controls for production
Staff Technical Training and Development
Meet and maintain training requirements
Develop and maintain personal development plan
Provide annual performance self-assessment on development plan
General Responsibilities:
Act as a role model (Lead by Example)
Act as a Subject Matter Expert SME for improvement projects
Act as a resource / SME for staff
Provide technical training for area personnel and assess training effectiveness
Develop training material for technical training
Assess staff skill sets and provide feedback to Supervisor
Perform scheduled cleaning of equipment
Assist in the assembly and disassembly of process equipment
Perform standardization of equipment
Perform basic 5S housekeeping
Initiate and follow up on Corrective Work Orders in the C3ME system or equivalent system
Support change over activities
Responsibility to adhere to any applicable EHS requirements.
Commitment to a fair and respectful relationship to others and behaviour in accordance with Takeda’s Code of Conduct.
Any other duties as assigned by supervisor
What you bring to Takeda:
Education and Experience Requirements
Degree in Chemical Engineering / Bioengineering / Chemical & Biomolecular Engineering / Pharmaceutical Engineering or related and possesses more than 4 years of relevant experience in the biotechnology or pharmaceutical industry.
Diploma in Applied Chemistry / Biomedical Sciences / Chemical Engineering / Food Science & Technology / Biologics & Process Technology / Chemical & Pharmaceutical Technology/ Food Science & Nutrition / Molecular Biotechnology / Biotechnology / Chemical & Biomolecular Engineering / Pharmacy Science or related and possess more than 6 years of relevant experience in the biotechnology or pharmaceutical industry
Nitec in Biotechnology / Chemical Process Technology or related with more than 8 years of relevant experience in the biotechnology, pharmaceutical industry.
Excellent self-motivated team player with hands-on attitude and good communication skills
Able to work on 12 hours rotating shift
Will work holidays and overtime as required
May be required to adjust work schedule to meet production demands
Key Skills and Competencies
Will be required to perform as a subject matter expert for equipment and/or systems
Knowledge of cGMP’s and applicable agency regulations (such as the FDA, EMEA) to ensure inspection readiness of department.
Proficient at following written instructions in the form of Electronic Batch Management (EBM), Batch Records (MBRs, MFRs) and Standard Operating Procedures (SOPs) where applicable
Self-motivated individual with the ability to complete and manage multiple floor activities in an effective and compliant manner. In the absence of the supervisor, they are the person of authority.
Expected to act on behalf of the supervisor while on the floor
Demonstrates appropriate level of adaptability, maintains positive outlook, and demonstrates composure under pressure.
Possess excellent communication skills to all levels throughout the organization
Possess excellent troubleshooting skills
Proficient documentation and proficient computer skills
Proficient in aseptic technique where applicable
Proficient as a system user of business systems such as C3ME and Trackwise
Ability to wear personal protective equipment such as safety glasses/goggles, gloves and safety shoes
Ability to gown and gain entry to biotechnology/pharmaceutical manufacturing areas.
Ability to work in and out of 2-8 deg C Cold Rooms with appropriate personal protective equipment if required
Ability to work in confined spaces if required
Ability to work around chemicals (alcohols, acids & bases)
More about us:
At Takeda, we are transforming patient care through the development of novel specialty pharmaceuticals and best in class patient support programs. Takeda is a patient-focused company that will inspire and empower you to grow through life-changing work.
Certified as a Global Top Employer, Takeda offers stimulating careers, encourages innovation, and strives for excellence in everything we do. We foster an inclusive, collaborative workplace, in which our teams are united by an unwavering commitment to deliver Better Health and a Brighter Future to people around the world.
Empowering our people to shine:
Takeda is proud in its commitment to creating a diverse workforce and providing equal employment opportunities to all employees and applicants for employment without regard to race, color, religion, sex, sexual orientation, gender identity, gender expression, parental status, national origin, age, disability, citizenship status, genetic information or characteristics, marital status, or any other characteristic protected by law.